Abstract
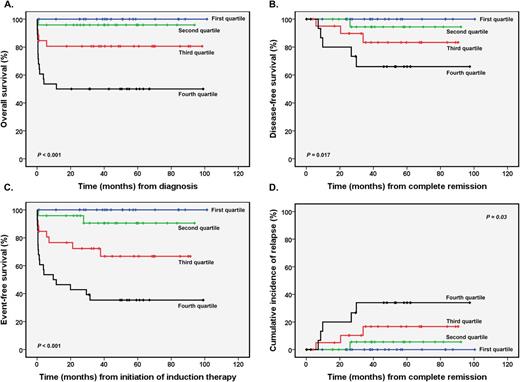
INTRODUCTION: In the context of all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy, heterogeneity of clinical outcomes of patients with acute promyelocytic leukemia (APL) may be higher than expected, mainly outside well-controlled clinical trials. Although recent clinical studies suggest that such heterogeneity could be significantly reduced or even abolished in light of the current arsenic trioxide (ATO)-based therapies, this compound is still not available for most reference centers in low- and middle-income countries (LMIC), and, at least for a near future, alternative strategies for predicting outcomes and improve risk stratification in APL should be tested. Here, we combined recurrent mutations with aberrant expression of genes previously associated with poor prognosis in both APL and acute myeloid leukemia, and proposed an integrative score in APL (ISAPL) for outcomes prediction. METHODS: As a learning set, diagnostic bone marrow samples from 150 adult patients with newly diagnosed APL who were enrolled in the IC-APL study were analyzed. The treatment protocol was identical to that of the LPA2005 trial reported by the Programa Español de Tratamiento en Hematologia/Dutch-Belgian Hemato-Oncology Cooperative Group (PETHEMA/HOVON), except for the replacement of idarubicin by daunorubicin due to its better availability and lower cost in the participating centers. Integer weights for the risk score were derived from Cox proportional hazard model, identical to that reported by Damm et al. (Blood. 2011 Apr 28;117(17):4561-8), using OS as primary endpoint. Variables with P -values lower than 0.05 were included in the model. Variables considered for the model inclusion were the following: FLT3 -ITD status, gene expression profile of ∆Np73/TAp73 ratio and transcript levels of KMT2E, BAALC, ID1, and WT1 genes. Other candidates, such as PIM2, PRAME, ERG, and IDH1 rs11554137 and WT1 rs16754 polymorphisms, were not associated with lower OS and not included in the score. Hazard ratios (HR) for OS were calculated for each variable separately. The HR was converted to integer weights according to the following: variables with HR < 1 were excluded from analyses; variables with HR > 1 and < 1.5 were assigned a weight of 1; variables with HR > 1.5 and < 2.5 were assigned a weight of 2; variables with HR > 2.5 were assigned a weight of 3. The final score was the sum of these integer weights. RESULTS: Complete data for ISAPL modeling were available for 99 of 150 patients (median score: 5, range: 0-16). The median age of the learning set was 35 years (range: 18-65 years) with 44 males (44%). According to PETHEMA/GIMEMA criteria for predicting relapse, 14%, 41%, 27% and 60% of patients assigned to the first, second, third and fourth ISAPL quartiles were deemed high-risk patients (P=0.036). Except for a higher leukocyte count at diagnosis in patients assigned to the fourth-ISAPL quartile (P=0.033), no significant differences in baseline features were found among groups. Early mortality (P=0.005), complete remission (P=0.002), overall survival (P <0.001; Figure 1A), disease-free survival (P=0.017; Figure 1B), event-free survival (P <0.001; Figure 1C), and cumulative incidence of relapse (P=0.03; Figure 1D) rates were significantly different among patients assigned to the first-, second-, third- and fourth-ISAPL quartiles. CONCLUSIONS: In summary, ISAPL resulted in the separation of four distinct groups of patients, with significant differences for hematologic recovery, relapse, and survival. We will next validate these findings in internal and external validation cohorts.
Bittencourt: Janssen, Takeda: Honoraria. Pagnano: Roche: Speakers Bureau; Amgen: Consultancy; Bristol-Meirs Squibb: Consultancy, Speakers Bureau; Novartis: Consultancy. Lo Coco: Novartis: Honoraria, Speakers Bureau; Lundbeck: Honoraria, Speakers Bureau; TEVA: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal